Beyond Heat: Non-Thermal Techniques for Vein Treatment
Within the medical specialty of phlebology, physicians and researchers are always seeking ways to improve vein care. Before we introduce the latest non-thermal techniques for vein treatment, let's define the two thermal techniques most commonly used in vein care.
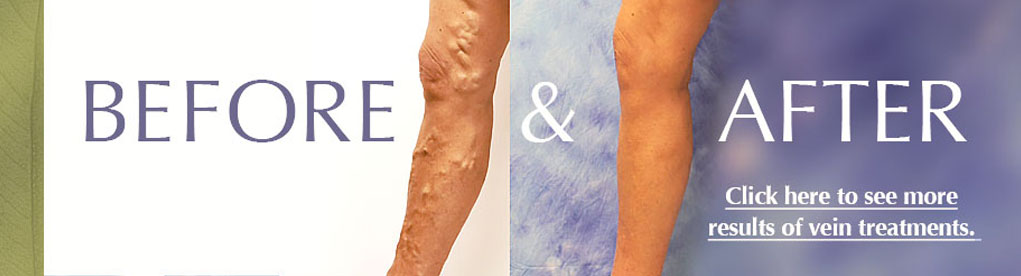
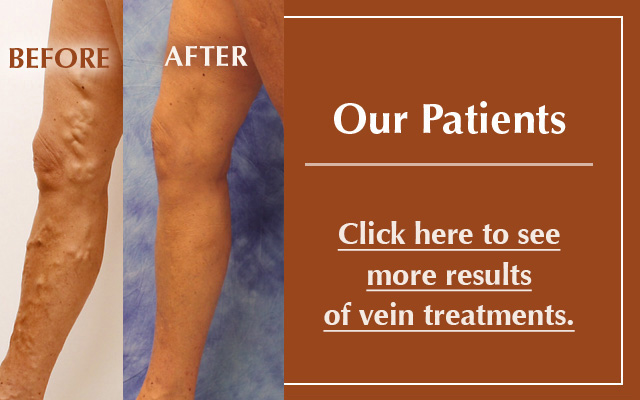
Our legs are comprised of a network of veins that are similar to branches on a tree: they contain large, or major veins, and increasingly smaller veins.. Healthy veins carry blood from all extremities back to the heart. In the legs, blood is usually traveling against gravity, thus the valves in the leg veins perform an important function. Venous insufficiency, or vein disease, occurs if the valves in the veins become damaged and allow the backward flow of blood in the legs towards the feet instead of the towards the heart.
When blood cannot be properly returned to the heart through the vein, the blood can "get backed up" or pool in the feet, leading to a feeling of heaviness and fatigue, causing congestion that will result in varicose veins and other skin changes. Over time, this increased pressure can cause additional valves to fail. If left untreated, it can lead to leg pain, swelling, ulcers, and other health problems.
Proper treatment means evaluating a patient's complete venous system, so that poorly functioning veins can be identified and treated at the source. Endovenous thermal ablation uses laser energy or radio waves to create an intense localized thermal reaction in the incompetent vein. This thermal energy causes the vein to seal shut, stopping the healthy blood flow from entering the damaged vein. This keeps the blood flowing toward the heart, not allowing it to change directions and return to the feet. The body will reabsorb the damaged and treated vein, forcing the blood to be diverted to healthy veins in the leg.
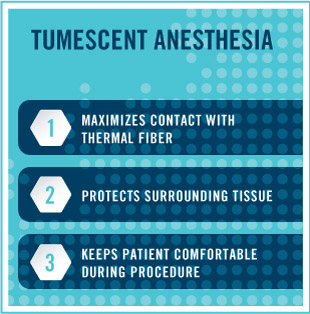
Endovenous thermal ablation, using laser or radio frequency, is an outpatient, minimally invasive procedure performed with a local anesthetic. It is considered the gold standard in treatment of the great and small saphenous veins. Part of the treatment involves tumescent anesthesia, a technique in which a high volume of a dilute local anesthetic is infiltrated around the vein.
Tumescent anesthesia serves three purposes during thermal ablation. First, the fluid causes the vein walls to collapse around the thermal fiber, maximizing contact. Second, the fluid creates an insulating ring around the vein and thermal energy source. This protects all surrounding tissues, including nerves and muscles, thus stopping any type of collateral damage. The third function is as an anesthetic, keeping the patient comfortable during the procedure. The tumescent fluid can be introduced in many ways, but at the hand of some physicians, the process can be very uncomfortable. Additionally, if there is epinephrine in the mixture, it can cause tachycardia and other unpleasant feelings of anxiety.
Non-thermal, non-tumescent (NTNT) modalities are newer procedures that do not require the use of tumescent anesthesia. The most talked-about NTNT techniques are currently: chemical ablation, mechanico-chemical ablation (MOCA), and cyanocryalate adhesives.
Standardized compound makes for safer foam
Sclerotherapy isn't a new treatment for treating troublesome veins. (Primitive versions of injecting a substance into veins to seal them up have existed since as early as the 17th century.) Modern day sclerotherapy is a process in which a medicine is injected into the vein causing it to collapse and seal itself shut. The body will then reabsorb the vein into local tissue, in effect making the problematic vein disappear, shunting the blood into the healthy collateral veins.
Foam sclerotherapy has been used widely in the Phlebology community in the past dozen years. The "Tessari method" has been the most common technique for producing foam for use in sclerotherapy. This technique involves manually mixing a small volume of liquid sclerosant with a volume of room air or other gas using two syringes and a three-way tap, or stopcock. Preparations created this way are referred to as "physician-compounded foam."
While physician-compounded, non-proprietary foam is inexpensive to make, medical experts caution that there are risks: there have been a number of published cases of micro-air emboli (small bubbles of gas in the bloodstream) from so-called "homemade foam" leading to neurological events in patients, including migraines, vision changes, strokes, and death. No physician-compounded foam has been approved by the U.S. Food and Drug Administration.
In 2013, the FDA did approve Varithena®, a chemical foam solution indicated to close the Great Saphenous Vein (GSV) and related tributaries. It is a proprietary mixture of 1% polidocanol, 65% oxygen, 35% carbon dioxide, and less than .8% of nitrogen. Dr. Robert Diamond, Vice President of Medical Affairs at BTG, the company that manufactures Varithena, explained what makes their product unique:
"When a physician creates his own foam, he uses room air, which can contain as much as 78% nitrogen—compared to Varithena, which has extremely low nitrogen," he explained. "While oxygen and carbon dioxide are rapidly absorbed into the blood stream, nitrogen is very slowly absorbed and those bubbles can persist and possibly cause a gas embolism and obstruct an important blood vessel."
Varithena's primary safety feature is the consistency of its bubbles: each one is between 100-500 microns, with uniform density and stability—a standardized compound that is much more predictable for vein doctors to use on their patients, compared with physician-compounded foam, which isn't subject to any manufacturing standards.
The company also goes to great lengths to standardize the use of Varithena, meticulously training every practitioner who plans to use it. According to Anastasia Mironova,
Vice President Marketing at BTG, clinically certified representatives conduct in-person training sessions about all aspects of the Varithena procedure. In addition, every Varithena sales representative must complete a rigorous certification process.
As more physicians in the U.S. have begun to administer Varithena to their patients, not all insurance companies have agreed to cover the cost, though that is changing.
"BTG has a team of people whose sole purpose is to grow coverage for Varithena, and they have made considerable progress with payers," said Mironova. "Seven of the top ten commercial payers cover Varithena, as well as 22 of the top 25 'Blues' plans. With over 165M lives covered, BTG is leading the way in reimbursement for the entire NTNT category."
BTG provides a number of services to help practices navigate the reimbursement process. The Varithena Solutions Center handles all the benefit verifications and pre-authorizations that are needed prior to treating patients. BTG also has a field-based team (Varithena Reimbursement Managers) that work directly with the practices to ensure that patients are adequately assessed by the insurance carriers.
Device uses unique design to deliver medicine
The ClariVein® Infusion Catheter (ClariVein-IC) is a system designed to introduce physician-specified agents into the peripheral vasculature. Developed by interventional radiologist Michael Tal, M.D. and John Marano, PhD (both co-founders of Vascular Insights, the company that manufactures ClariVein), the device was cleared by the FDA in 2008.
The ClariVein-IC is designed with a 360˚rotatable fluid dispersion wire connected to a proximally located integral battery powered motor drive unit. The catheter is introduced through a microintroducer at a single location, where the catheter sheath is navigated through the vein to the treatment site using ultrasound imaging. Physician-specified fluid is then delivered through its infusion port, surrounds the dispersion wire, and exits via an opening at the distal end of the catheter. The delivery of the fluid is enhanced by the use of the rotating dispersion wire to mix and disperse the infused fluid in the blood stream and on the vessel wall. In other words, the rotating inner wire agitates the epithelial lining of the vein, and when the fluid is sprayed from the top of tip of the catheter as it is pulled back, coverage is through and the effectiveness of the fluid is increased.
Many phlebologists are excited by the development of the infusion catheter and have described the use of it as "mechanico-chemical Ablation, or MOCA, in their research. Currently, however, the only FDA-approved device that stimulates the endothelium of a vein while introducing medicine is the ClariVein-IC.
Lindy McHutchison, MD, a physician at the Carolina Vein Center, has been using ClariVein in her practice for two years. She believes that there are a number of benefits to using the catheter in vein care. "Because there is no heat, you don't need a lot of anesthetic, plus you disrupt less tissue around the vein," said Dr. Hutchinson. "It is less painful, less inflammatory, and produces less swelling and bruising after the procedure."
Although ClariVein is still a relatively new device in the vein care specialty, several pivotal clinical studies using the ClariVein-IC system have been published, with data that were comparable with the results using EVLA methods. Insurance coverage for the ClariVein-IC device varies by geography and insurance company.
Vein closure using cyanocryalate adhesives
Cyanoacrylate, a kind of strong, fast-acting medical adhesive, that has been used in the treatment of blood vessel abnormalities (arterio-venous malformations) for over a decade. In February 2015, the FDA approved the VenaSeal® Closure System, which uses a medical adhesive to permanently treat varicose veins in the legs.
The VenaSeal system is made up of an adhesive, a specially formulated n-butyl-2-cyanoacrylate, and delivery system components that include a catheter, guidewire, dispenser gun, dispenser tips, and syringes.
During the procedure, the physician fills a syringe with the medical adhesive, and then inserts it into a dispensing gun attached to an application catheter. Using ultrasound imaging, the doctor inserts the catheter through the skin into the diseased vein to allow injection of the VenaSeal agent, a clear liquid that polymerizes into solid material. The catheter is placed in specific areas along the diseased vein and the physician conducts a series of trigger pulls to deliver the medical adhesive, thus sealing the incompetent venous valve and eliminating reflux. Compression is applied to the leg during the procedure, according to the indication for use by the FDA, but since it is not required post-procedure, VenaSeal is an attractive option for patients who cannot tolerate compression stockings.
Medical technology company Medtronic launched the VenaSeal closure system in the U.S. in November 2015. Currently, it is the only non-thermal, non-tumescent, non-sclerosant procedure approved for use in the U.S. (It is also available in international markets, including Hong Kong, South Africa and the United Arab Emirates.)
According to Mark A. Turco, MD, Medical Director of Medtronic Aortic and Peripheral Vascular, VenaSeal closures are performed in a minimally invasive outpatient procedure and produces minimal-to-no pain or bruising.
"The procedure is designed to minimize patient discomfort and reduce recovery time." said Dr. Turco. "Unlike other treatments, VenaSeal does not require the use of tumescent anaesthesia, minimizing the need for multiple needle sticks as compared to ablation procedures—and patients may not need to wear compression stockings post-procedure, although some patients may benefit from wearing them."
In terms of paying for the VenaSeal, no insurance companies cover the procedure and patients must pay out of pocket at this time.
Transforming current care
The current standard of care for venous insufficiency is vastly superior to the traditional surgical approaches they have replaced. Thermal ablation procedures have all the usual advantages of minimally invasive techniques over vein stripping—lower anesthesia requirements, faster recovery, lower risk of complication—and are very effective treatments. But, said Dr. Robert Worthington-Kirsch, Chairman of the American College of Phlebology CME Committee, "they aren't perfect."
According to Kirsch, MD, FSIR, FCIRSE, FACPh, RVT, RPVI, tumescent analgesia is probably the least pleasant component of these therapies, and probably accounts for much post-procedure bruising and discomfort, at least in the short term.
Dr. Kirsch has studied and considered non-thermal ablation techniques and their place in phlebology for some time.
"The emerging non-thermal techniques have the potential to make treatment of saphenous vein disease even less invasive, with much improved patient experiences," he observed. "If they prove to be effective and durable therapies, non-thermal ablation may be as transformative and disruptive to the current care of superficial venous insufficiency as thermal ablation has been to conventional surgery."
REFERENCES
Varithena:
T King, MD, et al. Efficacy and Safety Study of Polidocanol Injectable Foam for the Treatment of Saphenofemoral Junction (SFJ) Incompetence (VANISH-1). European Journal of Vascular and Endovascular Surgery. 2015;50:784–793.
Todd KL. The VANISH-2 study: a randomized, blinded, multicenter study to evaluate the efficacy and safety of polidocanol endovenous microfoam 0.5% and 1.0% compared with placebo for the treatment of saphenofemoral junction incompetence. Phlebology. 2014 Oct;29(9):608-18. doi: 10.1177/0268355513497709.
ClariVein:
S Elias, Raines JK. Mechanochemical tumescentless endovenous ablation: final results of the initial clinical trial. Phlebology. 2012 Mar;27(2): 67–72. doi: 10.1258/phleb.2011.010100
Boersmaa D, et al. Mechanochemical Endovenous Ablation of Small Saphenous Vein Insufficiency Using the ClariVein® Device: One-year Results of a Prospective Series. European Journal of Vasc and Endovasc Surg. 2013 Mar:45(3):299–303.
VenaSeal:
T Lane, et al. Cyanoacrylate glue for the treatment of great saphenous vein incompetence in the anticoagulated patient. Journal of Vasc Surg. 2013 July:1(3):298-300.
N Morrison, et al. Randomized trial comparing cyanoacrylate embolization and radiofrequency ablation for incompetent great saphenous veins (VeClose). Journal of Vasc Surg. 2015 Apr:61(4):985–994
This article was featured in Vein Health News, a publication founded by Dr. Cindy Asbjornsen to educate medical professionals and patients about vein health.
Dr. Asbjornsen is the Editorial Director for Vein Health News, Maine's vein magazine for primary care physicians. Read the Latest Issue and Subscribe to Vein Health News.
![]() Do I have Venous Disease?
Do I have Venous Disease?
Millions of people across the United States experience its symptoms. Find out more by asking yourself these 12 questions.
 Request an Appointment
Request an Appointment
Ready to take the next step towards understanding your vein health and the available treatment options? Click here to request an appointment.